Press release
Peripheral IV Catheter Market is Expected to Progress at a CAGR of 5.8% to Reach US$ 1,865.4 Million by 2034 | Fact.MR Report
The peripheral IV catheter market in US is predicted to account for 88.6% of the North American market in 2024. It is projected that the US market for peripheral IV catheters would grow by US$ 685 million by 2024. By 2034, the market is expected to reach US$ 1,029 million with a 4.2% compound annual growth rate.It is projected that the size of the peripheral IV catheter market worldwide would reach US$ 1,058.6 million by 2024. By 2034, the market is expected to grow at a 5.8% CAGR and reach US$ 1,865.4 million.
𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐚 𝐒𝐚𝐦𝐩𝐥𝐞 𝐂𝐨𝐩𝐲 𝐨𝐟 𝐓𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭: https://www.factmr.com/connectus/sample?flag=S&rep_id=9654
For public relations (PR) specialists, the peripheral I.V. catheter market offers a distinctive environment with a wealth of chances for strategic communication and brand development. We will examine the dynamics, difficulties, and future growth potential of the Peripheral IV Catheter market in this study, highlighting the critical role public relations plays in influencing attitudes, building connections, and propelling commercial success. The global peripheral I.V. catheter market is expected to rise steadily over the next several years due to a mix of ongoing technology advancements, greater environmental consciousness, and an increasing demand for more efficient operations. It is expected that industry participants would focus on product innovation, strategic partnerships, and global expansion in order to take advantage of the changing market prospects.
𝐓𝐨𝐩 𝐃𝐫𝐢𝐯𝐞𝐫𝐬 𝐏𝐫𝐨𝐩𝐞𝐥𝐥𝐢𝐧𝐠 𝐆𝐫𝐨𝐰𝐭𝐡 𝐢𝐧 𝐭𝐡𝐞 𝐏𝐞𝐫𝐢𝐩𝐡𝐞𝐫𝐚𝐥 𝐈𝐕 𝐂𝐚𝐭𝐡𝐞𝐭𝐞𝐫 𝐌𝐚𝐫𝐤𝐞𝐭
Peripheral IV catheter sales are benefiting from an aging population and an increase in the prevalence of chronic illnesses.
The market is being driven by a rise in the number of hospitalizations and a notable use of safety-ported short peripheral intravenous catheters.
Peripheral IV catheter use is predicted to be significantly accelerated by the desire to reduce needle-stick injuries and growing healthcare costs.
Peripheral IV catheter use is rising as more people become aware of the benefits of early diagnosis in obtaining blood samples for testing.
Peripheral intravenous catheter use is rising as a result of the rise in chronic illnesses like diabetes, neurological diseases, infectious disorders, etc. that are creating opportunities for players to conduct disease treatment awareness campaigns.
The market is expected to grow as a result of investments made in the development and production of effective peripheral IV catheters, given the growing patient population.
𝐂𝐨𝐦𝐩𝐞𝐭𝐢𝐭𝐢𝐯𝐞 𝐋𝐚𝐧𝐝𝐬𝐜𝐚𝐩𝐞
Key players in the peripheral IV catheter market include Becton, Dickinson, and Company, B. Braun Melsungen AG, Smiths Group plc., Terumo Corporation, Venner Medical, Vygon, Teleflex Incorporated, C. R. Bard, Inc., NIPRO Medical Corporation, and Argon Medical Devices, Inc.
There are both domestic and foreign competitors in this fiercely competitive market. Small and medium-sized businesses are focusing on increasing their market share by investing in technology innovations. Players also use other inorganic and organic tactics, like as alliances, mergers, acquisitions, and the introduction of new products.
Recent Developments in the Peripheral IV Catheter Market
In September 2022, B. Braun Medical Inc. purchased numerous items from Starboard Medical. These devices are used to fasten the Clik-FIX peripheral catheter.
In March 2022, Shockwave Medical Inc. announced that the Shockwave M5+ peripheral IVL catheter-which received FDA certification and the CE Mark-will be made commercially accessible globally.
In July 2022, B. Braun Medical Inc. introduced the Introcan Safety 2 IV Catheter, which allows for single-time blood control. The most recent invention from B. Braun lowers the risk of needlestick injuries and blood exposure while also improving the safety quotient of IV for medical professionals.
𝐆𝐞𝐭 𝐂𝐮𝐬𝐭𝐨𝐦𝐢𝐳𝐚𝐭𝐢𝐨𝐧 𝐨𝐧 𝐭𝐡𝐢𝐬 𝐑𝐞𝐩𝐨𝐫𝐭 𝐟𝐨𝐫 𝐒𝐩𝐞𝐜𝐢𝐟𝐢𝐜 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡 𝐒𝐨𝐥𝐮𝐭𝐢𝐨𝐧𝐬: https://www.factmr.com/connectus/sample?flag=RC&rep_id=9654
𝐒𝐞𝐠𝐦𝐞𝐧𝐭𝐚𝐭𝐢𝐨𝐧 𝐨𝐟 𝐏𝐞𝐫𝐢𝐩𝐡𝐞𝐫𝐚𝐥 𝐈𝐕 𝐂𝐚𝐭𝐡𝐞𝐭𝐞𝐫 𝐌𝐚𝐫𝐤𝐞𝐭 𝐑𝐞𝐬𝐞𝐚𝐫𝐜𝐡
By Product Type:
Power Injected PICC
Conventional PICC
By Design Type:
Single Lumen
Double Lumen
Multiple Lumen
By End User:
Hospitals
Ambulatory Surgical Centers
Catheterization Labs
By Region:
North America
Europe
Latin America
East Asia
South Asia and Oceania
Middle East and Africa
𝐂𝐨𝐧𝐭𝐚𝐜𝐭:
US Sales Office
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583, +353-1-4434-232
Email: sales@factmr.com
𝐀𝐛𝐨𝐮𝐭 𝐅𝐚𝐜𝐭.𝐌𝐑
We are a trusted research partner of 80% of fortune 1000 companies across the globe. We are consistently growing in the field of market research with more than 1000 reports published every year. The dedicated team of 400-plus analysts and consultants is committed to achieving the utmost level of our client's satisfaction.
This release was published on openPR.
Permanent link to this press release:
Copy
Please set a link in the press area of your homepage to this press release on openPR. openPR disclaims liability for any content contained in this release.
You can edit or delete your press release Peripheral IV Catheter Market is Expected to Progress at a CAGR of 5.8% to Reach US$ 1,865.4 Million by 2034 | Fact.MR Report here
News-ID: 3482260 • Views: …
More Releases from Fact.MR
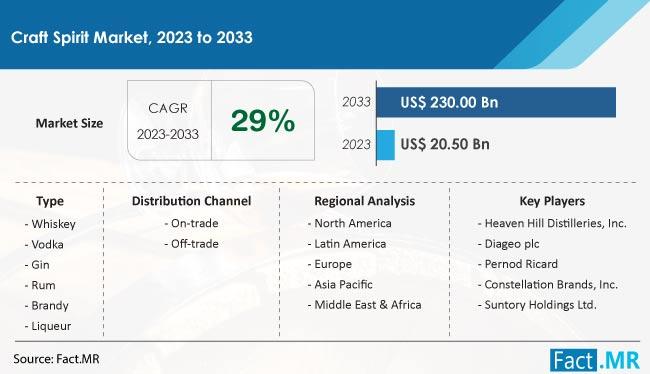
Craft Spirit Market Anticipates $ 59.47 Billion Valuation by 2034, Driven by 29% …
According to the most recent market analysis conducted by knowledgeable analysts at Fact.MR, the size of the worldwide craft spirit market is estimated to be worth US$ 20.5 billion in 2023 and is expected to grow at a CAGR of 29% to reach a valuation of US$ 230 billion by the end of 2033.
Craft spirits are growing in popularity because of their exceptional flavor, quality, and distinctiveness. The craft spirits…
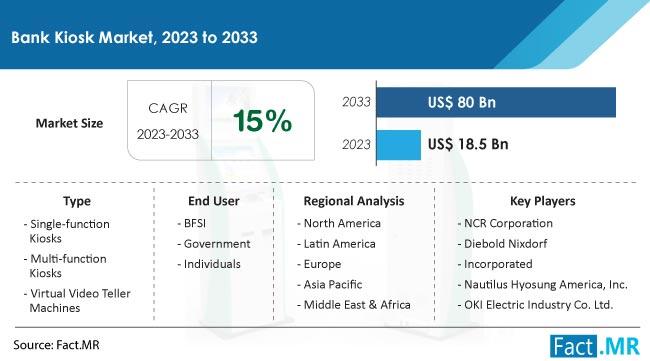
Bank Kiosk Market Forecasted to Expand Rapidly, Projecting US$ 80 Billion Value …
A recent analysis by knowledgeable experts at Fact.MR values the worldwide bank kiosk market at US$ 18.5 billion in 2023 and projects it will grow at a CAGR of 15% to reach US$ 80 billion by the end of 2033.
An automated self-service device called a bank kiosk offers customers a range of banking services without the need for human support. A vital component of the contemporary banking infrastructure, bank kiosks…
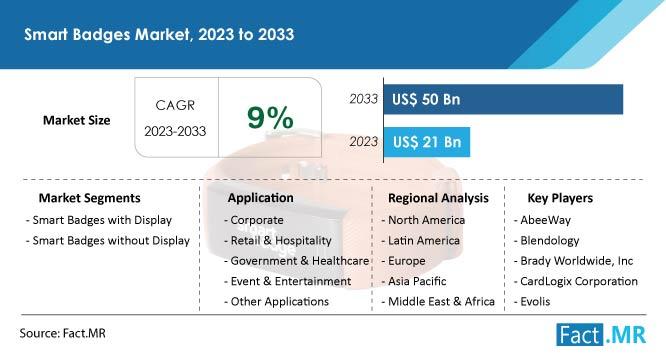
Smart Badges Market To Witness Exponential Growth, Expected to Hit US$ 50 Billio …
The smart badge market is expected to grow at a remarkable compound annual growth rate (CAGR) of 9% from 2023 to 2033, from its estimated valuation of US$ 21 billion in 2023 to US$ 50 billion by 2033.
An electrical gadget with an embedded memory or microcontroller in the shape of a touch pad is called a smart badge. It establishes a connection with a reader by short-range wireless connectivity or…
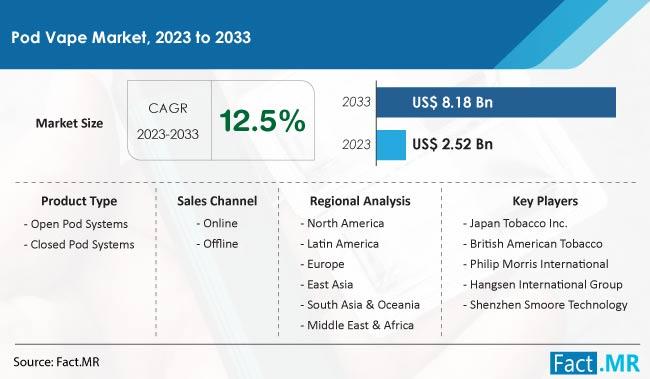
Pod Vapes Market Is Forecasted To Increase At A CAGR Of 12.5% By 2033: Fact.MR R …
The global pod vape market, valued at USD 2.52 billion in 2023, is projected to grow significantly, reaching USD 8.18 billion by 2033. This expansion represents a compound annual growth rate (CAGR) of 12.5% over the decade.
The Pod Vape Industry sales study offers a comprehensive analysis on diverse features including production capacities, Pod Vape demand, product developments, sales revenue generation and Pod Vape market outlook across the globe.
market research report…
More Releases for Peripheral
PC Gaming Peripheral Market
PC Gaming Peripheral Market Research study which offers insights of in-depth research on historic and current market size along with the expected future prospects of the market and emerging trends in the market. The PC Gaming Peripheral Market report provides crucial information about the market, including Opinions from Industry experts, and the recent progressions and developments of the PC Gaming Peripheral Market.
PC gaming peripheral are external hardware devices such as…
Peripheral Vascular Devices Market Report 2018: Segmentation by Types (Periphera …
Global Peripheral Vascular Devices market research report provides company profile for Abbott Vascular, Bayer Healthcare, Volcano Corporation, Teleflex Medical and Others.
This market study includes data about consumer perspective, comprehensive analysis, statistics, market share, company performances (Stocks), historical analysis 2012 to 2017, market forecast 2018 to 2025 in terms of volume, revenue, YOY growth rate, and CAGR for the year 2018 to 2025, etc. The report also provides detailed segmentation…
Peripheral Vascular Device Market Size, Peripheral Vascular Device Market Share, …
Global Peripheral Vascular Device Market Size is observed to gain traction owing to the factors such as increasing research and development for developing several new product, and rising funding by the private organizations.
Request for Sample of This Research Report @ https://bit.ly/2xjOKpC
Top Key Player:-
Abbott Laboratories
Braun Melsungen AG
Boston Scientific Corporation
R. Brad, Inc.
Cardinal Health, Inc.
Medtronic plc.
Cook Medical, Inc.
Teruma Corporation
Jude Medical, Inc.
The Spectranetics Corporation
Volcano Corporation
Peripheral vascular disorder (PVD) is a blood circulation disorder…
Peripheral Artery Disease Market By Type (Peripheral Angioplasty Balloons, Perip …
Peripheral artery disease is characterized by plaque built up in the arteries carrying blood from heart to legs, arms, and other limbs. Peripheral artery disease in turn also increases the risk of other cardiovascular disorders such as heart attack, coronary heart disease, stroke, and ischemic attack.
Request Sample At: https://www.bigmarketresearch.com/request-sample/1633533
The global peripheral artery disease market generated $3,136 million in 2016, and is projected to reach $4,980 million by 2023, registering a…
Peripheral Intravenous Catheter Market
New research report offers a comprehensive analysis of the “Peripheral Intravenous Catheter Market: Asia Pacific Regional Market to Witness the Fastest Growth Throughout the Forecast Period: Global Industry Analysis & Opportunity Assessment, 2016-2021“The main objective of this report is to deliver insightful information and clear-cut facts pertaining to the growth trajectories of the market.
Request to view Sample Report @ https://www.mrrse.com/sample/2827
Peripheral intravenous catheter is a sophisticated medical device widely used to…
Peripheral Arterial Disease (PAD)/ Peripheral Vascular Disease (PVD) - Pipeline …
Market Research Hub's Pharmaceutical and Healthcare latest pipeline guide Peripheral Arterial Disease (PAD) Peripheral Vascular Disease (PVD) - Pipeline Review, H1 2017, provides comprehensive information on the therapeutics under development for Peripheral Arterial Disease (PAD) Peripheral Vascular Disease (PVD) (Cardiovascular), complete with analysis by stage of development, drug target, mechanism of action (MoA), route of administration (RoA) and molecule type. The guide covers the descriptive pharmacological action of the therapeutics,…